Abstract
Background: Sickle cell disease (SCD) is an inherited group of red blood cell disorders with a complex pathophysiology largely driven by vaso-occlusion and hemolytic anemia. Vaso-occlusive crises (VOCs) are the hallmark of SCD. VOCs and continuing silent vaso-occlusion are associated with cycles of inflammation and tissue injury that can lead to acute and chronic organ complications.
Sickle cell nephropathy (SCN) is the term used to describe kidney-related complications of SCD; manifestations include hyperfiltration, albuminuria and progressive loss of kidney function. Endothelial dysfunction may play a role in the development of albuminuria in SCD. Increased plasma endothelin-1 (ET-1), a biomarker of endothelial dysfunction, is significantly associated with an increase in the urine albumin-to-creatinine ratio (ACR) (Ataga et al. PLoS One 2016). Patients (pts) with SCN may develop chronic kidney disease (CKD; diagnosed when abnormalities in kidney structure or function persist for >3 mo). Pts with SCD and baseline (BL) albuminuria ≥100 mg/g can develop persistent albuminuria, which is associated with a rapid decline in estimated glomerular filtration rate (eGFR) (Niss et al. Blood Adv 2020).
No treatments are approved for CKD due to SCN; current SoC usually consists of angiotensin-converting enzyme inhibitors (ACEI), angiotensin-receptor blockers (ARBs) and/or hydroxyurea/hydroxycarbamide (HU/HC).
P-selectin is one of the drivers of multicellular adhesion leading to vaso-occlusion. Preclinical studies have shown that P-selectin expression increases in response to renal ischemia-reperfusion injury (Zizzi et al. J Pediatr Surg 1997). Crizanlizumab, a humanized monoclonal antibody that binds to P-selectin, significantly reduced the median annualized VOC rate vs placebo (Ataga et al. NEJM 2017). The aim of the Phase II STEADFAST study (NCT04053764) is to determine the effect of P-selectin inhibition with crizanlizumab on kidney function in pts with SCD and early-stage CKD.
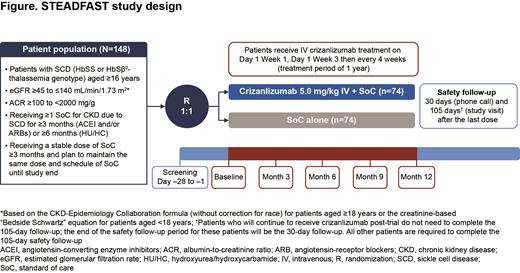
Methods: The STEADFAST trial aims to enroll 148 pts aged ≥16 yrs with CKD due to SCD; pts eligible for inclusion are detailed in the figure. Pts will be randomized 1:1 to receive crizanlizumab 5.0 mg/kg (intravenous infusion over 30 min on Day 1 of Wk 1, Day 1 of Wk 3, then every 4 wks for 1 yr) and their usual SoC or their usual SoC alone. Pts will be stratified based on CKD risk category (moderate risk or high/very high risk, based on eGFR and albuminuria assessed by ACR) and if they are receiving HU/HC. Exclusion criteria include pts with a history of stem cell transplant, evidence of acute kidney injury within 3 mo of study entry, or those receiving renal replacement therapy.
The primary endpoint is the proportion of pts with ≥30% decrease from BL in ACR at 12 mo. A logistic regression model including treatment effects and stratification factors will be utilized; the test (based on the log-odds ratio estimated by the model) will be performed at a 1-sided significance level of 0.025. Secondary endpoints include mean change in ACR from BL to 3, 6, 9 and 12 mo, the percentage change in eGFR from BL to 3, 6, 9 and 12 mo, the proportion of pts with a protein-to-creatinine ratio (PCR) improvement (≥20% decrease) or stable PCR (within ±20%) at 12 mo compared with BL and the proportion of pts with progression of CKD (based on pre-defined eGFR decline and ACR increase) from BL to 12 mo. To minimize variability of ACR measurements, 3 urine samples (2/3 samples collected as morning voids) will be collected at all designated timepoints. Exploratory endpoints include assessments of renal and cardiac biomarkers, including ET-1 and soluble P-selectin, at BL, 3, 6, 9 and 12 mo, echocardiography at BL and 12 mo and renal MRI at BL, 6 and 12 mo (at selected sites).
Results: As of July 2021, 31 pts have been enrolled, with 36 sites in 10 countries currently open to enrollment. Open sites by country include USA (9 sites), Brazil (5 sites), Spain (4 sites), Italy, Turkey and the UK (3 sites each), France, Greece and Panama (2 sites each), Lebanon, the Netherlands and South Africa (1 site each). Study sites in Bahrain, Egypt, Ghana and Kenya are planned and Ireland, Tanzania and Saudi Arabia have been recently added as new participating countries.
Conclusion: The Phase II STEADFAST study has been designed to evaluate whether crizanlizumab, in combination with SoC, can provide a potentially targeted disease-modifying benefit for pts with SCD and CKD. The trial is open for enrollment.
Saraf: Pfizer: Research Funding; Global Blood Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding. Ataga: Forma Therapeutics: Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees; Agios Pharmaceuticals: Consultancy; Novartis: Membership on an entity's Board of Directors or advisory committees; F. Hoffmann-La Roche Ltd: Consultancy; Global Blood Therapeutics: Membership on an entity's Board of Directors or advisory committees. Derebail: Travere Therapeutics: Consultancy; Novartis: Consultancy; Bayer: Consultancy; UpToDate: Patents & Royalties. Sharpe: Napp Pharmaceuticals: Speakers Bureau; Novartis Pharmaceuticals: Consultancy. Lebensburger: Novartis: Consultancy; Bio Products Laboratory: Consultancy. DeBonnett: Novartis Pharmaceuticals Corporation: Current Employment. Zhang: Novartis: Current Employment. Bartolucci: INNOVHEM: Other: Co-founder; Hemanext: Consultancy; AGIOS: Consultancy; Jazz Pharma: Other: Lecture fees; GBT: Consultancy; Emmaus: Consultancy; F. Hoffmann-La Roche Ltd: Consultancy; Bluebird: Consultancy, Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Lecture fees, Steering committee, Research Funding; Fabre Foundation: Research Funding; Addmedica: Consultancy, Other: Lecture fees, Research Funding.
The presentation will discuss use of crizanlizumab as an investigational therapy in patients with sickle cell disease and sickle cell nephropathy

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal